Sterile Lyophilized Vials
Looking for small scale cGMP lyophilization services?
Dalton offers high quality lyophilization services which include cycle optimization and cGMP production for clinical scale batches.
Our cGMP clinical scale lyophilizer can handle batch sizes aligned with your early phase clinical needs. The 0.56 m2(6.12 ft2) lyophilizer can accommodate vial sizes from 2ml to 10ml, with a capacity of approximately 1,900 vials for the 3ml format or 1,100 vials for the 5 ml format.*
Our new 18 ft2 lyophilizer on stream in 2016 will enable us to meet the demand for larger batch sizes. It will be able to accommodate from 6936 vials (2 ml format), 5,400 vials (3 ml), 3,564 vials (5ml), 2,376 vials (10ml) and 680 vials (20ml).
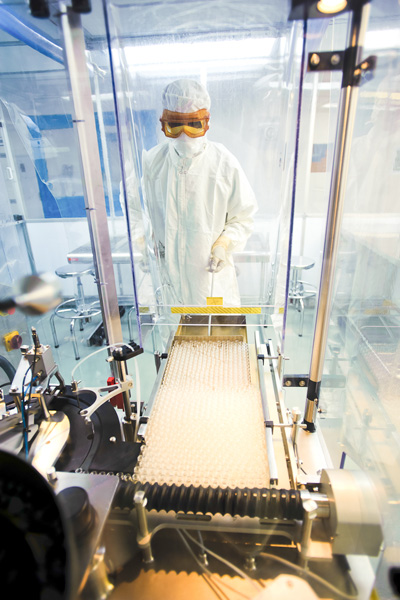
Dalton uses its upgraded sterile filling room to aseptically fill the vials and then transfers them to our fully programmable cGMP lyophilizer. Our experts can aseptically fill and lyophilize your large molecule biologic or small molecule in complete compliance.
Lyophilized vials are inspected, tested and released in-house via our well-equipped analytical and microbiology laboratories.
Bulk vials can be labelled with basic single color labels at Dalton so you are ready for the clinic. Bulk material lyophilization is also available at Dalton.
* Quantities vary by vial size
