
Extractables and Leachables Testing Services
What are Extractables and Leachables?
Extractables are defined as organic and inorganic contaminants such as plasticizers, elastomers, oligomers, dyes, elemental impurities etc. that can be extracted from the surfaces of packaging components under extreme conditions such as elevated temperature and pressure or with exposure to organic solvents.
Leachables are defined as organic and inorganic contaminants that can be released from the surface of container closure systems under standard storage conditions.
Extractables and leachables (E&Ls) may be inherently toxic and can contaminate the drug product. To ensure the safety and efficacy of drug products there has been an increase in regulatory measures to monitor and control extractables and leachables in pharmaceuticals, drug delivery systems, and biomedical devices.
The enhanced regulatory standards necessitates pharma industry to develop sensitive and accurate analytical methods to detect, identify, and quantitate extractables and leacheables in drug product, as per U.S. FDA 21 CFR 211.94(a) and European Commission Directive (2001/83/EC).
Extractables and leachables represent contaminants from a broad range of structural categories, covering inorganics (elemental impurities Hg, Pb, Cd, As, etc…), organics (PAHs, Nitrosamines, Azo Dyes, Vulcanizing Agents, Phthalates, antioxidants, Elastomers, Stabilizers, etc…) with a wide range of molecular weights and physico-chemical properties.
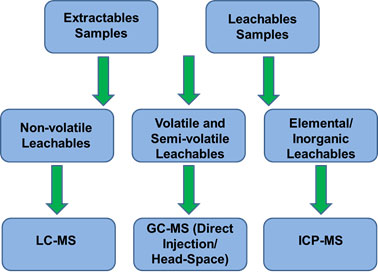
No single analytical technique in the study of extractables and leacheables can be used for all categories of compounds. As a result, testing for extractables and leachables is achieved by complementary use of a variety of analytical techniques, as shown below:
- HPLC-UV/ELSD/CAD
- LC-MS/MS (ESI, APCI)
- GC-FID/TCD
- GC-
- ICP-MS
- FT-IR
- Thermoanalysis TGA, DSC
Dalton Pharma Services offers consultation services and customized testing capabilities for determining extractables and leachables on container closure systems.
- Determination of the Analytical Evaluation Threshold (AET) based on Safety Concern Threshold (SCT) and dose per container.
- Development of tailored study designs for extractable and leachables, including profiling (inorganic and organic) and simulated leachable studies.
- Validation of analytical methods developed for determination of extractables and leachables on container closure systems.
- Routine analysis of extractables and leachables in drug product by chromatographic and spectroscopic techniques
- Development of analytical methods to evaluate Chemical and structural changes (e.g. by DSC) in polymeric container closure systems by spectroscopic or thermal analysis.
Regulations:
There has been a significant amount of regulation and guidance for industry for extractables and leachables study, with a large percentage of the requirements established over the last few years.
Extractable and leachable studies are essential for the identification and quantitation of toxic leachable impurities that can migrate from pharmaceutical container closure systems, process equipment and packaging, resulting in adulterated products. Extractable and leachable study is an important and essential parameter, especially for high risk sterile Parenteral and Ophthalmic Drug Products.
A successful Extractable and leachable study using validated analytical methods assures that the quality of drug product is not compromised in container closure system; ensuring patient safety.
Useful Links:
- Dalton’s Whitepaper on Extractables and Leachables
- https://www.dalton.com/qara
- Guidance for Industry: Container Closure Systems for Packing Human Drugs and Biologics
- https://www.ema.europa.eu/en/documents/regulatory-procedural-guideline/directive-2001/83/ec-european-parliament-council-6-november-2001-community-code-relating-medicinal-products-human-use_en.pdf
- EudraLex Volume 4, Chapter 3
- Q3D
- https://pqri.org/