
White Paper: Drug Repurposing: A Smart Drug Development Strategy
Published May 2023
Drug Repurposing – An Overview
Drug repurposing has emerged as an effective strategy for meeting unmet medical needs and advancing personalized medicine. It involves finding new uses for existing drugs to provide more effective treatments and develop affordable drugs. It is a potential solution to the challenges faced by traditional drug discovery methods, such as high R&D costs, long timelines, low success rates, and regulatory hurdles4.
Repurposing strategies can be drug-oriented or disease-oriented, and drug-oriented approaches can be on-target or off-target repurposing. Different approaches to drug repurposing include phenotypic screening-based approach, literature-based approach, chemical similarity-based approach, and molecular similarity/signature-based approach. Successful drug repurposing often incorporates both drug-oriented and disease-oriented approaches.
Drug repurposing has the potential to provide lower cost development of drugs, more effective treatments, and medicines with favorable side effect profiles, especially for neglected diseases as well as rare and orphan indications. Additionally, AI & network-based analysis can be used to identify potential drug targets and predict the effects of drug candidates on the network. This approach can also be used to identify drugs that can be combined with existing treatments for better efficacy or reduced side effects.
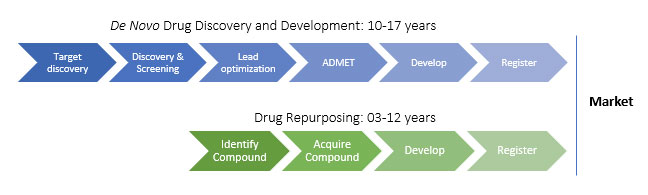
While drug repurposing offers a promising alternative to traditional drug discovery methods, it is not without its challenges. The challenges include the need for robust and reliable data, the need for innovative approaches to identify new uses for existing drugs, and the need for regulatory approval for new indications. Despite these challenges, drug repurposing has already shown success in several cases, including the repurposing of thalidomide for the treatment of multiple myeloma and lenalidomide for the treatment of myelodysplastic syndromes.
While drug repurposing offers a promising alternative to traditional drug discovery methods, it is not without its challenges. The challenges include the need for robust and reliable data, the need for innovative approaches to identify new uses for existing drugs, and the need for regulatory approval for new indications. Despite these challenges, drug repurposing has already shown success in several cases, including the repurposing of thalidomide for the treatment of multiple myeloma and lenalidomide for the treatment of myelodysplastic syndromes.
With continued innovation and investment, drug repurposing has the potential to transform the field of medicine and improve outcomes for patients around the world.
Drug repurposing: Financial Considerations
The process of applying existing treatments for new therapeutic uses has emerged as a unique value proposition for the investors, patients, and payers. The rising and unsustainable costs in drug development and the consequent drug cost inflation are contentious issues on global agendas and a concern for the industry. It is predicted that by 2020, $1.4tn will be spent globally on prescription drugs, and healthcare payers are seeking to curb expenditures while ensuring access to high-quality care10. Drug repurposing brings forth the benefit of quickening patient access to innovative and effective treatment at lower risk and development costs for the industry. Furthermore, with an estimated cost of repurposing of $8.4m, a fraction of the R&D costs for a new therapy, drug repurposing can translate into revenues by extending product life cycles11.
Recent technological developments in data mining, machine learning, and artificial intelligence open new avenues for developing targeted drug repurposing approaches. By building on the knowledge that drugs with similar chemical and molecular profiles can potentially have similar targets for diseases with similar pathways and gene activities, "repurposing analytics" emerge as powerful tools to optimize the identification of suitable drug candidates as well as predicting drug-disease interactions.
While there are 4,000 APIs approved globally, many of these molecules have been strategically shelved. These molecules can be made available to other companies or research institutes for further investigation of their potential use in alternative indications12. Moreover, with more than 80% of the 30,000 available drugs being ex-patent, substantial drug repurposing market opportunities exist for drug companies, university research groups, and new start-ups13.
However, the regulatory landscape is becoming more complex and stringent in public and private healthcare systems. In addition, the current value assessment frameworks for reimbursement schemes do not contemplate the possibility of maximizing value through finding 'new homes' for existing drugs, and there are currently no streamlined processes to assess the value of repurposed drugs or to adapt them to scale in healthcare systems. This remains an area of opportunity for regulatory agencies to assist industry and provide the regulatory framework to support drug repurposing initiatives.
Drug Repurposing: A Strategic Fight Against COVID -19
Repurposing existing or abandoned drugs is a desirable way to quickly find a treatment and potential cures for emerging diseases like COVID-19. To facilitate such efforts, the World Health Organization (WHO) established a research network to identify drug therapies and combinations that could be used to treat COVID-195. Past examples of repurposed drugs include minoxidil, sildenafil (ViagraTM), and thalidomide. In addition, repurposing drugs are more budget-friendly and faster than developing a drug from scratch. Patents covering drugs are also crucial to business strategy, attracting investors by fostering cross-licensing opportunities and further collaboration.
A new and non-obvious innovation for existing drugs must be identified to obtain a patent for drug repositioning. This could include alternative crystal forms, salt forms, formulations, alternative delivery methods, stereoisomers, or deuterated analogues. The drug must also have the potential as a therapeutic to treat the new indication (i.e. COVID-19). A repurposed drug must be filed as a New Drug Submission (NDS) with Health Canada in Canada6. In the United States, a relevant regulatory approval pathway for drug repurposing is section 505(b)(2)7, 8. This provision allows the applicant to rely upon studies the Federal Drug Administration (FDA) previously considered in evaluating the drug’s safety. In Europe, a similar approval pathway is available through the European Medicines Agency (EMA) under Article 10a of Directive 2001/83/EC9.
Several open-source platforms have been set up to support initiatives dedicated to repurposing drugs to treat and cure COVID-19. These platforms provide an excellent opportunity to share and identify potential drug repurposing candidates which are already undergoing safety, efficacy, and toxicity studies for treating different diseases, thereby presenting pharmaceutical companies with new markets. Repurposing drugs is further incentivized by granting intellectual property (IP) protections, such as patents and in the case of orphan indications, periods of exclusivity.
Artificial Intelligence in Drug Repurposing
Artificial intelligence (AI) is helping accelerate drug discovery by extracting hidden patterns and evidence from biomedical data3. AI approaches include literature extraction, machine learning from genomic and bioinformatics data, and mining electronic medical records and billing data. These data-driven approaches cover everything from drug-protein interactions at the molecular level to sifting through millions of records to find drugs used to treat other conditions.
AI has the potential to reduce drug development time and costs, ultimately accelerating the delivery of life-saving treatments to patients with rare diseases and cancer. Traditional drug discovery and development models are time-consuming, costly, and fraught with a high risk of failure. Only 10% of new drug applications1 obtain market authorization. In contrast, 30% of repurposed drugs2 are licensed for new indications, giving companies market-based incentives to repurpose existing assets.
These systematic approaches to repositioning old medicines are disease-centric, goal-centric, and drug-centric. A disease-centric system identifies close relationships between new and old indications. A goal-centric approach connects a known goal and its established drug to a unique indication. Finally, drug-centric approaches link known drugs to new targets and their associated indications.
A challenge4 faced by repurposing old drugs is the existing data packages may not meet current standards. Some studies may have enrolled only a few patients and need more statistical power. Despite these challenges, drug repurposing continues to gain popularity as advanced AI opens the door to new insights into drug targets in disease and increases the likelihood of successful clinical development trials.
Conclusion:
The global drug repurposing market was worth USD 31.3 billion in 2020 and is expected to reach USD 46.9 billion by 2028 with a CAGR of 5.4% over the forecast period18. By finding new uses for existing drugs to provide more effective treatment options, repositioning strategies provide alternatives for industry to offer cheaper alternatives, more effective treatments as well as drugs with favorable side effects, especially for neglected and rare diseases and orphan indications. It offers a promising additional strategy to traditional drug discovery methods, & with continued innovation and investment, drug reuse can transform the field of medicine and improve outcomes.
References:
- Source: DiMasi, J. A., Grabowski, H. G., & Hansen, R. W. (2016). Innovation in the pharmaceutical industry: New estimates of R&D costs. Journal of health economics, 47, 20-33.
- Ashburn, T. T., & Thor, K. B. (2004). Drug repositioning: identifying and developing new uses for existing drugs. Nature Reviews Drug Discovery, 3(8), 673-683.
- IQVIA, a global healthcare analytics company, uses innovative AI and machine learning techniques" - Source: IQVIA website: https://www.iqvia.com/solutions/commercialization/real-world-evidence/drug-repurposing.
- Pushpakom, S., Iorio, F., Eyers, P. A., Escott, K. J., Hopper, S., Wells, A., & Pirmohamed, M. (2019). Drug repurposing: progress, challenges and recommendations. Nature Reviews Drug Discovery, 18(1), 41-58.
- Aitken, M., and Kleinrock, M., Global medicines use in 2020: outlook and implications. IMS Institute for healthcare informatics, 2015;1–4
- Pdersiis, A. (2011), The benefits of drug repositioning. Available at: http://www.ddw-online.com/business/p142737-the-benefits-of-drug-repositioning-spring-11.html
- Shim, J., and Liu, J., International Journal of Biological Sciences, 2014;10(7):654–663.
- Kollewe, J. (2012). Why finding new uses for old drugs is a growing business. [online] the Guardian. Available at: https://www.theguardian.com/business/2012/nov/27/new-uses-old-drugs-business
- https://www.who.int/emergencies/diseases/novel-coronavirus-2019/global-research-on-novel-coronavirus-2019-ncov/solidarity-clinical-trial-for-covid-19-treatments
- https://www.canada.ca/en/health-canada/services/drugs-health-products/drug-products/fact-sheets/drugs-reviewed-canada.html
- 21 USC § 355 (2012)
- 21 USC § 355(b)(2) (2012)
- https://www.ema.europa.eu/en/documents/regulatory-procedural-guideline/european-medicines-agency-pre-authorisation-procedural-advice-users-centralised-procedure_en-0.pdf
- Aitken, M., and Kleinrock, M., Global medicines use in 2020: outlook and implications. IMS Institute for healthcare informatics, 2015;1–4
- Persidis, A. (2011), The benefits of drug repositioning. Available at: http://www.ddw-online.com/business/p142737-the-benefits-of-drug-repositioning-spring-11.html
- Shim, J., and Liu, J., International Journal of Biological Sciences, 2014;10(7):654–663.
- Kollewe, J. (2012). Why finding new uses for old drugs is a growing business. [online] the Guardian. Available at: https://www.theguardian.com/business/2012/nov/27/new-uses-old-drugs-business
- https://growthmarketreports.com/report/drug-repurposing-market-global-industry-analysis